Understanding the difference between sodium-ion and lithium-ion batteries
 Dec 28,2023
Dec 28,2023

 Basen
Basen
Introduction
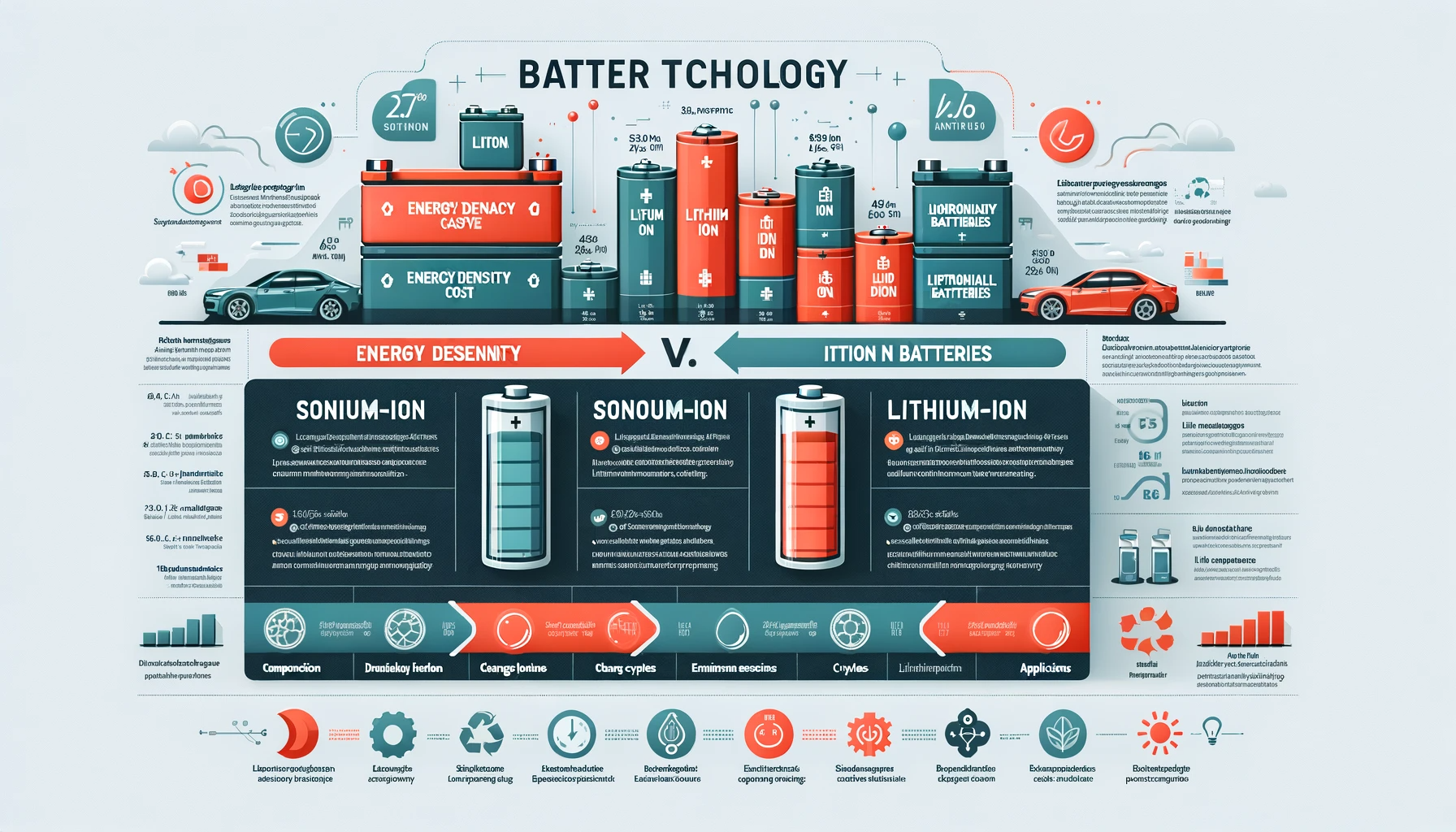
In the realm of rechargeable batteries, lithium-ion (Li-ion) batteries have been the dominant choice for decades, powering everything from smartphones to electric vehicles. However, with the advent of sodium-ion (Na-ion) batteries, there's a growing interest in understanding how they differ from their lithium-ion counterparts. This blog delves into the key differences between sodium-ion and lithium-ion batteries, providing insights into their composition, performance, and applications.
Composition and Working Principle
Lithium-Ion Batteries: Li-ion batteries are composed of a lithium compound as the cathode material and a graphite anode. They operate on the principle of moving lithium ions between the anode and cathode during charging and discharging cycles.
Sodium-Ion Batteries: Na-ion batteries, on the other hand, use sodium compounds as the cathode material. They function similarly to Li-ion batteries, with sodium ions shuttling between the electrodes. However, the larger size of sodium ions compared to lithium ions influences the battery design and material choices.
Energy Density and Efficiency
Lithium-Ion: Li-ion batteries are known for their high energy density, which means they can store more energy per unit of weight. This makes them ideal for applications where size and weight are critical factors, such as in mobile devices and electric vehicles.
Sodium-Ion: Na-ion batteries typically have a lower energy density than Li-ion batteries. While this may limit their use in energy-intensive applications, they are still suitable for stationary storage applications like grid storage, where size and weight are less of a concern.
Cost and Availability
Lithium-Ion: The production of Li-ion batteries involves rare and costly materials like cobalt and lithium, whose availability is concentrated in specific parts of the world. This contributes to the higher cost of Li-ion batteries.
Sodium-Ion: Sodium is abundant and widely available, making Na-ion batteries potentially cheaper to produce. This could lead to more affordable energy storage solutions, particularly in regions where lithium is not readily available.
Environmental Impact
Lithium-Ion: The mining and processing of lithium and other metals used in Li-ion batteries have significant environmental impacts, including water pollution and habitat destruction.
Sodium-Ion: Na-ion batteries are considered more environmentally friendly due to the abundant availability of sodium and the absence of heavy metals in their composition.
Charge Cycles and Longevity
Lithium-Ion: Li-ion batteries generally offer a higher number of charge cycles and longer overall lifespan, which is crucial for applications like electric vehicles.
Sodium-Ion: While Na-ion batteries have shown promising cycle life in early research, their long-term durability compared to Li-ion batteries is still being evaluated.
Applications
Lithium-Ion: Widely used in portable electronics, electric vehicles, and renewable energy systems.
Sodium-Ion: Emerging as a viable option for stationary energy storage, backup power systems, and potentially for low-cost, low-energy-density applications.
Conclusion
While lithium-ion batteries currently lead the market in terms of energy density and widespread use, sodium-ion batteries present an exciting alternative, particularly in terms of cost, environmental impact, and resource availability. As research and development in Na-ion technology continue to advance, we can expect to see these batteries playing a more significant role in various energy storage applications, complementing the existing Li-ion technology.






 HOME
HOME The Red Sea Incident and the Future of Battery Prices in 2024
The Red Sea Incident and the Future of Battery Prices in 2024  You May Also Like
You May Also Like

 Tel
Tel
 Email
Email
 Address
Address